| MEDICAL INTRO |
| BOOKS ON OLD MEDICAL TREATMENTS AND REMEDIES |
THE PRACTICAL |
ALCOHOL AND THE HUMAN BODY In fact alcohol was known to be a poison, and considered quite dangerous. Something modern medicine now agrees with. This was known circa 1907. A very impressive scientific book on the subject. |
DISEASES OF THE SKIN is a massive book on skin diseases from 1914. Don't be feint hearted though, it's loaded with photos that I found disturbing. |
THE CHEMISTRY OF ALCOHOL AND OF ALCOHOLIC BEVERAGES |
|||||
21 |
|||||
“ Beer is a far more dangerous enemy to Germany than all the armies of France.”—Von Moltke.
“Alcohol is a waste-product in the activity of the yeast plant.”1— C. F. Hodge, Ph.D., Professor of Physiology, Clark University. |
||||||
1 Physiological Aspects of the Liquor Problem.
22 |
||||||
CHAPTER II |
|||||
THE CHEMISTRY OF ALCOHOL AND OF ALCOHOLIC BEVERAGES
When in everyday life we talk of alcohol, we think either of one particular substance, or more often, perhaps, of the drinks or beverages of which the most conspicuous property—that of causing intoxication—is too well known. All these beverages possess one point in common, namely, that of containing more or less of a powerful chemical substance named alcohol, or, more properly, “ethyl alcohol.” For instance, beer is a drink containing about 5 parts of alcohol in every 100 parts of beer, i.e. 5 per cent. The composition of beer is, roughly, as follows :—
Water ........ 90 per cent.
Albumin ....... 0.5 ,,
Sugar ........ l.5 ,,
Mineral ........0.4 ,,
Extractive ........ 3.1 ,,
Alcohol ........ 4.5 ,,
——
100
The different alcoholic beverages may be classified into three groups : (1) beers ; (2) wines ; (3) spirits or distilled liquors.
1. Beers.—The principal beverages belonging to this class are porter, stout, and various beers, such as lager beer,—with a percentage of alcohol of 4 to 7 per cent.1
2. Wines.—Belonging to the second class we have port wine, sherry, claret, champagne, and home-made wines. In ordinary wine the amount of alcohol is somewhat greater than the amount in beer,—varying from 9 to 22 per cent. In home-made wines, such as “ currant,” “ raspberry,” “ elderberry,”
1 The stronger beers go up to 10 per cent, see evidence before the Departmental Committee on Beer Materials, 1899.
23 |
|||||
24 ALCOHOL AND THE HUMAN BODY chap.
“cranberry,” “orange,” “gooseberry,” or “rhubarb” wine, between 5 and 12 per cent of alcohol is found.
3. Spirits.—The liquids of the third class—brandy, whisky, and other spirits—contain 40 to 56 per cent of alcohol.
The amount of alcohol contained in certain well-known alcoholic beverages is as follows :— |
||||||||
Percentage of Alcohol.
Beer . . . . . . . 4 to 5 per cent (by weight).
Cider, Perry, and other home-made
wines . . . . . . 5 to 10 ,,
Hock, Claret . . . . . . 8 to 11 ,,
Port . . . . . . . 16 to 18 ,,
Marsala . . . . . . . 14 to 24 ,,
Orange Wine, Raspberry Wine . . . 10 to 12 ,,
Champagne . . . . . . . 8 to 11 ,,
Sherry, Madeira . . . . . . 13 to 18 ,,
Gin . . . . . . . . about 31 ,,
Rum, Strong Liqueurs . . . . . 40 to 50 ,,
Whisky . . . . . . . 44 to 50 ,,
Brandy . . . . . . . 48 to 56 ,,
Rectified Spirit . . . . . . 84 ,,
Methylated Spirit1 . . . . . . 90 ,, (by volume). |
||||||||
It must be clearly understood that, in all the beverages above mentioned, alcohol is present—the amount being only a matter of proportion. In a pint of ale there are two table-spoonfuls of alcohol; while in a pint of wine there are about six tablespoonfuls, and a pint of brandy consists of about equal parts of alcohol and water.
PREPARATION OF ALCOHOL
Alcohol may be prepared in a number of ways, but all that we need remember for practical purposes is that it is obtained as a rule from the fermentation of sugars. Starchy materials are also used, these starchy materials being utilised in order to provide the sugar needed for fermentation.
Fermentation
Fermentation is a common process with which we are familiar in everyday life. It is the process by which milk
1 Methylated Spirit, which is largely used for burning in spirit lamps, and in the preparation of different kinds of wood polish, consists of about 90 per cent of ethyl alcohol and 10 per cent crude wood spirit (impure methyl alcohol), together with a small quantity of naphtha added for the purpose of rendering the spirit undrinkable. |
||||||||
THE CHEMISTRY OF ALCOHOL 25 |
|||||
becomes sour, butter turns rancid, fruit decomposes, and beer is formed from malt. In all these cases a chemical change takes place, and we have to consider how this change is caused. When milk is left standing exposed to the air, it turns sour without anything apparently being added. But the air is full of minute forms of vegetable life—“ micro-organisms “—which, although so small that they can only be seen with a powerful microscope, are extremely active. They produce something which is called a ferment or “ enzyme,” and it is due to this ferment that the chemical change called fermentation takes place. Therefore, in order to prevent milk or various other substances from fermenting, it must be kept away from the air or anything containing these micro-organisms.
The fermentation by which alcohol is produced is chiefly brought about by an airborne micro-organism called the yeast plant, growing in the presence of sugars, as will be explained later on. This yeast plant produces a ferment which acts especially on certain sorts of sugar, splitting them up into alcohol and carbon dioxide (carbonic acid) gas. Fermentable sugars, i.e. sugars capable of being thus split up, are found in many situations and many substances, for instance, in grapes, in apples, and in barley-grains at a certain period of their growth.
In the case of grapes the micro-organisms which produce the right kind of ferment to turn the grape sugar into alcohol, gather from the air and collect upon the outside of the grape. While there, they cannot attack the juice,—but as soon as the grapes are crushed and squeezed into a pulp and the skins are broken, the micro-organisms begin to grow and increase very rapidly, at the same time producing their ferment, which splits up the sugar in the grapes into alcohol and carbon dioxide gas, bubbles of which escape freely.
We have mentioned the sugar which exists in grapes ; and we must now describe another kind of sugar which is found in sprouting barley, and can also be turned into alcohol, provided that there be present the ferment which is produced by the micro-organism called yeast.
Description of Yeast
Yeast is a microscopical plant, consisting of a single cell. It grows and multiplies very rapidly by budding when placed |
|||||
ALCOHOL AND THE HUMAN BODY |
|||||||||||||||||||||
26 |
|||||||||||||||||||||
CHAP. |
|||||||||||||||||||||
in a warm sugary solution such as “sweet-wort,” and ferments the sugar, breaking it up into alcohol, water, and carbonic acid. But, strange to say, there is a definite limit to the growth and
multiplication of the |
|||||||||||||||||||||
 |
|||||||||||||||||||||
yeast plant, for alcohol of a certain strength hinders its activity. When first placed in the sugary solution the yeast thrives vigorously ; bubbles of carbonic acid (car bon dioxide) gas rise to the surface and alcohol collects in the vat. Gradually it |
|||||||||||||||||||||
shows less sign of |
|||||||||||||||||||||
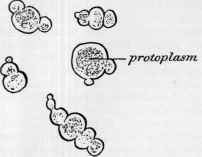
Fig. 4.—Yeast cells in active growth and budding. The protoplasmic centre of each segment is shown as a granular material. |
|||||||||||||||||||||
vigour, and finally, when the alcohol |
|||||||||||||||||||||
present in the solu tion reaches 13 per cent, the growth and multiplication of the yeast ceases, being checked and finally arrested by the presence of alcohol in the surrounding fluid. Thus the alcohol is not a source of either energy or food to the yeast plant, but on the contrary is injurious to it and stops its growth. This remark able fact is in accordance with recent “findings” of science as to the inhibiting effect of alcohol upon plant growth, to which we shall refer in the next chapter.
Sources of Sugar used in the Manufacture of Alcohol
The most interesting part of the processes involved in the preparation of alcohol is undoubtedly the method adopted in order to obtain large supplies of cheap sugary liquids in which yeast has the power of growing and therefore of converting them into alcohol.
Fortunately for the brewer there is provided in nature a store of such sugar, which is meant to supply growing seeds with nourishment, during the period of growth which occurs before their rootlets are developed and are able to obtain their food from the soil. A grain of barley in the dry state consists largely of insoluble starch lying in contact with the tiny |
|||||||||||||||||||||
THE CHEMISTRY OF ALCOHOL |
||||||||||||||||
27 |
||||||||||||||||
II |
||||||||||||||||
embryo plant. Now, as soon as the grain becomes moist and warm and ready to sprout or germinate, this stored-up starch begins to alter (under the action of an agent present in many |
||||||||||||||||
plants known as “diastase”) |
 |
|||||||||||||||
into a soluble form of sugar. This is needed as the food-supply for the germinating plant, and if undisturbed the little barley germ thrives for some time on this sugar, until it is vigorous enough to send out rootlets into the soil and thus become an independent plant capable of growth and development.
Now the first part of the art of brewing consists in starting this process of germination; then in waiting a few days until the grain has developed its diastase and part of the conversion of starch into sugar has occurred; and then in |
||||||||||||||||
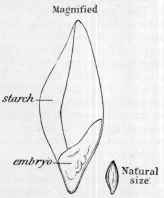
Fig. 5.—Two drawings of a barleycorn. The small one on the right shows the grain of its natural size. The large drawing is the same grain magnified to show that it mainly consists of starch, by the fer |
||||||||||||||||
mentation of which sugar and alcohol
suddenly checking the process are produced.
of development by overheating
the growing grains in a kiln, thereby arresting their growth
so that they shall not use up the sugar. This is known as
“ malting.”
The next process consists in grinding and breaking up these sprouting grains of malt, so that all the rest of the starch can be acted on by the diastase, which is so powerful that it is able to convert into sugar a certain amount of further additional starch if this be added to the solution. The resultant liquid is called “ sweet-wort,” and from this the residual grains are now separated. After being dried they are used as food for cattle.
When yeast is added to the sweet-wort, the soluble sugar it contains is rapidly converted into alcohol, carbonic acid gas, and water, as already explained on p. 25.
Finally, the liquid containing the alcohol and water is put through various processes. Sometimes it is heated and the alcohol driven off with a view to its separation and the prepara- |
||||||||||||||||
28 ALCOHOL AND THE HUMAN BODY chap.
tion of “spirit.” On the other hand, if beer is required, certain other preliminary processes are carried out,—for example, such things as “hops,” or one of its substitutes, are boiled with the “ sweet wort “ before fermentation is set up.
The practical details as to the manufacture of these beverages are very many, and would be out of place here; the one point of general interest being the simple fact that beer is prepared from the fermentation of malt grain, but also in very large quantity from other less desirable substances, and that to this solution other materials, for various reasons, are frequently added. The exact proportions of these and the chemical processes through which they are passed are secrets known to the trade—but it is notorious that in the manufacture of beer as carried on today in England, malt substitutes, hop substitutes, and various chemicals for preserving the beer are frequently used.
In the House of Commons, May 23, 1905, the Chancellor of the Exchequer reported, as a result of the Inland Revenue Analysis during the preceding two years, that in eighty cases objection was raised by the Government to the articles used as brewing materials on some one of the following grounds :—
(1) That the samples examined showed the presence of arsenic beyond the limit recommended by the Royal Com mission on Arsenical Poisoning ; (2) the presence of copper ; (3) the presence of quillaia bark or extract; or (4) that there were alcoholic flavouring essences.
The evidence given before the Departmental Committee on Beer Materials (1899) is indisputable, and may be here quoted.1
MATERIALS REFERRED TO BY PREVIOUS WITNESSES AS USED BY BREWERS
(Separated into Groups.) Malt and its Substitutes
Malt, corn, unmalted corn, raw grain, grain (other), maize, maize (flaked), maize (gelatinised), maizone, cerealine, sago (ground), torrefied malt, Duttson’s malt flour, Beane’s grist, Shepherd’s corn malt, rice, rice shells, rice flaked, rice gelatinised, rice desiccated, rizine, sugar, saccharum, glucose, glucose from sago, glucose from raw grain, glucose from maize, glucose from potato starch, glucose and gelatine, glucosine,
1 Minutes of evidence taken before the Departmental Committee on Beer Materials, 1899, p. 381. |
||||
THE CHEMISTRY OF ALCOHOL 29 |
|||||||||||||
11 |
|||||||||||||
molasses, raw sugar, cane sugar, honey, viscosoline, dextrine, malto-dextrine, black malt sugar, saccharin. Saccharin (coal-tar product) is not allowed : it is strictly forbidden.
Hops and its Substitutes Hops, quassia, Colombo root, camomiles, hop substitutes.
Chemicals Vitriol, salt, bisulphite of lime, salicylic acid,1 magnesia, tannin, sulphuric acid, chalk.
Colouring Matters Colouring, caramel, caramelised dextro-maltose, dextrinous caramel.
Clarifying Matters Finings, isinglass, fishy matter (sole skins).
Sundry and Stimulating Liquorice, grains of Paradise, Guinea pepper, Coceulus indicus. Cocculus indicus is also not permitted to be used.
The incessantly repeated efforts of some members of Parlia ment to obtain legislative power to stop this method of brewing from refuse and other substances have all failed in view of the influence of the drink traders.
It is stated that 1,868,000 acres of land in the United Kingdom are under barley cultivation for brewing purposes,— producing annually 65,000,000 bushels of barley; and that 19,000,000 cwts. of foreign barley are also used annually in this country for the production of alcohol. Now it takes about 3 1/5 lbs. of barley to make a gallon of ale,2 and the solid matter in a gallon of ale amounts to but half a pound, of which only a small part, i.e. the sugary and albuminoid portion, can be called nutritious. Therefore it appears that the conversion of nourishing barley into
(1) A small amount of nutritive material, and
(2) A larger amount of non-nutritious material—(alcohol,
extractives, etc)
1 “ It appears to be a common custom to preserve ale and beer by the addition of salicylic acid. The use of this drug for this purpose is every where recognised as harmful and unjustifiable.”—Report of the Massachusetts Board of Health, 1894. |
|||||||||||||
water, about 9 lbs.
alcohol, ½ lb.
extractives and salts, 4 to 5 oz.
maltose, 2 to 3 oz.
albuminoids, ½ to ¾ oz. |
|||||||||||||
2 One gallon of ale consists of |
|||||||||||||
30 ALCOHOL AND THE HUMAN BODY chap.
is, economically speaking, a matter of great waste, and so long as England contains human beings in need of cheap food, it is a waste that should be strongly discouraged by the nation.
Distillation
Thus far we have dealt only with the conversion of sugary liquids into alcohol, and have shown that where the alcohol present reaches 13 per cent no further alcohol can be formed by the yeast cells. Therefore, in order to obtain alcohol of a strength more potent than 13 per cent, another process known as “ distillation” has to be followed.
The principle is simple, i.e. that of heating a fermented liquid so that the alcohol (the lightest portion) is driven off in the form of vapour, most of the water being left behind. This vapour is collected and condensed again to the liquid condition by means of passing it through a long coil or “distiller,” which is cooled by a water jacket.
At first this “ distillate ” always contains some water in addition to the alcohol, but, if needs be, it is quite easy to repeat the process several times, and in this way to obtain pure alcohol practically free from water.
Malt whisky is prepared from malt and yeast by fermenta tion as described,—the liquid containing alcohol being finally run into a “still” and “distilled,” forming raw whisky.
For making such distilled liquors as whisky, gin, or “ Schnapps,” the starch is obtained from rye, maize, or oats, or from potatoes or beetroot.
Originally whisky (or at any rate Scotch whisky) was manufactured solely from barley malt, and this is still the case with some of the whisky distilled in the Highlands in pot-stills. At a moderate computation, roughly two-thirds of the spirit vended nowadays as “whisky” is derived from other materials, chiefly maize (Indian corn) and refuse molasses. The spirit obtained is (or should be) called “grain” or “patent” spirit, the word “grain” referring to the materials, and the qualification “patent” to the type of apparatus in which this variety of alcohol is distilled. This spirit, made from different materials by a different process, has “by-products” that, as might have been antici pated, are different. . . . Nevertheless this new spirit is sold as whisky both at home and in the Colonies, and is used for blending with malt whisky, the blend being in some instances so labelled as to give the purchaser the impression that it is malt whisky. ... It is known in the trade that much of the so-called whisky most carelessly made from the cheapest materials is exported to West Africa and other tropical |
||||
THE CHEMISTRY OF ALCOHOL 31 |
|||||||
II |
|||||||
colonies, where it is sold under Government sanction to native races.— (Extracted from the Report of the Whisky Commission instituted by the British Medical Association.)1
In the preparation of “ patent “ spirit various artificial means are used. For instance, alcohol may even be obtained from sawdust, which is first converted by means of acids into a fermentable sugar, which is then fermented. In addition to the recognised “still” and patent spirit of commerce, there are on the market certain cheap spirits artificially concocted from alcohol, prepared from inferior material and carelessly purified, and containing sometimes, besides the alcohol, other ingredients of a harmful nature.
The Massachusetts Board of Health reports (1894) that tannic acid was found in excess in 5 out of 37 samples of whisky.
Fusel oil in noxious amount is occasionally to be found, and it is also rarely present to a distinctly appreciable extent in beer.
Rum is made from a mixture containing molasses, which is fermented and then distilled.
Gin is made in practically the same way as whisky, but the distilled liquor is in addition redistilled with juniper berries, turpentine, coriander seed, or a variety of other flavouring materials.
WINES
Wines differ from spirits or distilled liquors in that they are prepared by the fermentation of fruit juices, chiefly the fruit of the vine. For many centuries this was the only source of wine, and even now people generally imagine that grapes form the basis of the ordinary wines of commerce, although it is well known that there have not always been sufficient grapes grown in Europe to supply the quantity of wine that is drunk. At one time, owing to the ravages of the phylloxera, other ways of providing wine had to be invented, and various methods came into vogue : it became, in fact, a fine art to combine alcohol with coloured liquids (turmeric, logwood, and other dyes being used), and these decoctions, being duly flavoured, were labelled as “ wine.”
Since the brilliant discovery of Pasteur, whereby the de struction of the vines was stopped, the demand for these 1 See Journal of British Medical Association, December 26, 1903. |
|||||||
32 ALCOHOL AND THE HUMAN BODY chap.
made-up wines has been less urgent; but, nevertheless, we need to be on the alert as regards their existence, because some of them are more intoxicating than ordinary wines, containing as they do a somewhat larger percentage of alcohol than exists in wine prepared from grape juice alone.
Unfermented Wines
The ancients appear to have used as a drink the fresh juice of the grape which had not been put through the process of fermentation. “ And Pharaoh’s cup was in my hand ; and I took the grapes, and pressed them into Pharaoh’s cup ” (Gen. xl. 11). This quotation with its context seems to show that the juice of fresh grapes was frequently enjoyed in the unfermented state, and proves therefore that when the word “ wine ” is used by ancient writers it does not necessarily refer to an alcoholic beverage, although undoubtedly fermented wines were also in use. In the present day unfermented wine is prepared on a large scale in Switzerland, Australia, and other places, according to one or more simple methods.
Method of preparing Unfermented Wine. — The grape juice, before it has had time to ferment, is heated to a certain point and then placed in sterilised vessels, which are sealed so that no micro-organism can enter. Hence, as no fermentation occurs, alcohol is not formed.
Grape juice can also be prevented from fermenting by other methods, e.g. :—
(1) Application of cold. (Grape juice will not ferment
at a temperature below 5° C. or 40° F.)
(2) By addition of antiseptics, such as salicylic, boracie,
sulphurous, benzoic, and cinnamic acids.
It must be noted in passing that certain products labelled “ unfermented wines ” are made in a somewhat different way, whereby a little alcohol is added to ensure their “keeping.”
This addition of alcohol to liquids which are nominally non-alcoholic takes place to no small extent in the preparation of patent medicines and drinks. It very seriously adds to the amount of alcohol consumed by the nation, an addition which various Governments are beginning to realise and taking steps to prevent. |
||||
But first, if you want to come back to this web site again, just add it to your bookmarks or favorites now! Then you'll find it easy!
Also, please consider sharing our helpful website with your online friends.
BELOW ARE OUR OTHER HEALTH WEB SITES: |
Copyright © 2000-present Donald Urquhart. All Rights Reserved. All universal rights reserved. Designated trademarks and brands are the property of their respective owners. Use of this Web site constitutes acceptance of our legal disclaimer. | Contact Us | Privacy Policy | About Us |